Polymer Lithium Ion Batteries Research in Multimedia University
The battery converts chemical energy into electrical energy through electrochemical reaction. The common lithium ion batteries consist of an anode (graphite) and a cathode (lithium cobalt oxide) separating by electrolyte. There are two common types of battery, i.e. primary and secondary. A secondary or rechargeable battery is a battery system which can be recharged and used many times. The first commercially available rechargeable lithium ion batteries are introduced by Sony Corporation in year 1991 where favorable response is attained in the energy storage market. Among the great challenges in the twenty-first century, energy storage and conversion had always been interested in the scientific and engineering studies. Innovative, inexpensive and environmentally friendly energy conversion and storage systems are essential in order to fulfil the demand of modern society and ecological concerns. Commercialization of lithium ion batteries in year 1991 by Sony Corporation attained a favourable response in the energy storage market due to its beneficial performance for portable electronic devices, such as laptop computers, smart phones, e-book readers, implantable medical instruments and etc. Furthermore, it also exhibits important application prospects as electrochemical energy storage system for electric vehicles and renewable energy. However, some concerns have arisen from the usage of organic solvent electrolytes, which include relatively confined stability domain that prevents the utilization of high voltage cathodes, safety issues due to high vapour pressure and flammability property of organic solvent, and manipulation hazards due to incompatibility with the human health and the environment.

High Capacity Polymer Li-Ion Battery
SEM micrographs of PEO-based electrolyte film with SiO2 <10 μm
Solid-state batteries are not quite ready for mass-market where researchers are still in their efforts to find a better solid material with higher and better overall performance at ambient temperature. Hence, more efforts are still required in order to replace the organic solvent electrolyte solutions with more reliable and safer electrolyte systems. Solvent-free lithium conducting polymeric membrane (polymer electrolyte) is one of the alternate approaches. The benefits offered by the lithium ion batteries, such as lightweight design, long cycle life, higher energy density, wide temperature range of operation, lower self-discharge rate and the absence of memory effects have enable their use in a wide variety of applications. Some important requirements of polymer electrolyte for the application in lithium ion batteries include adequate ionic conductivity, higher cation transference number, better interphase characteristics of electrolyte-electrode, ease of preparation and appropriate chemical, thermal and mechanical stabilities. However, it is not easy to find a polymer electrolyte that can fulfil all these requirements.
In late 2009, a polymer lithium ion battery (Poli-battery) project was initiated at Solid State Electronic Laboratory (SSE), Multimedia University (MMU), Malaysia. The proposed Poli-battery consists of PEO polymer, Li salts and fillers (SiO2) sandwiched between a cathode and anode to form a cell. The Poli-battery is designed to operate at low voltage with high power in order to optimize the operating cost. The construction of the Poli-battery was completed in early 2011. A series of testing and measurements has been carried out to verify the performance of the polymer battery. At the end of 2013, Poli-battery prototype have been presented at TM R & D, Cyberjaya. High quality Poli-batteries were obtained which demonstrated the capabilities of the Poli-battery for electronic appliances in Malaysia.

PMMA Samples
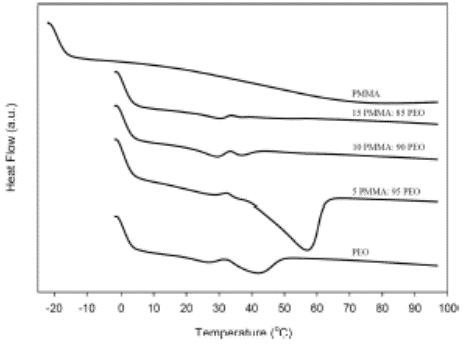
DSC thermograms of PMMA–PEO-blend solid polymer electrolytes
In our projects, poly(ethylene oxide) (PEO) and poly(methyl methacrylate) (PMMA) based solid polymer electrolytes were prepared by solution cast method. The effect of inorganic fillers on solid polymer electrolytes were investigated in these work incorporating the different filler types (i.e. silicon dioxide (SiO2), aluminium oxide (Al2O3) and titanium oxide (TiO2)) and different particle sizes (range of micron to nanometer). All the solid polymer electrolytes were studied and characterized using Electrochemical Impedance Spectroscopy (EIS), Fourier Transform Infrared (FTIR), X-ray Diffraction (XRD), Differential Scanning Calorimetry (DSC) and Scanning Electron Microscopy (SEM). The incorporation of inorganic fillers into PEO based solid polymer electrolyte systems is found to enhance the ionic conductivity. For PMMA based solid polymer electrolyte systems, optimum result is obtained with the addition of SiO2 fillers, where highest ionic conductivity is 2.612 × 10−4 Scm−1, compared with the filler-free PMMA polymer electrolyte, 4.272 × 10−6 Scm−1. In addition, inorganic fillers with smaller sizes are found to yield better results in the ionic conductivity. This enhancement is due to the interaction between the polar surface group on the fillers with cations and anions, which promotes the creation of additional sites to form favourable high conducting pathways for the migration of ions. These results are in agreement with the XRD, DSC and SEM results.
Recently, SSE laboratory has started the development of a PMMA-based Poli-battery for electric vehicle in Malaysia as electric vehicle is the most commonly vehicles in future. It is a series of polymer batteries to generate the high electrical power to operate the electric vehicles for daily operation. The polymer battery is mounted on electric vehicle which travels for both short and long distances. The polymer battery will be fabricated and tested in our laboratory in MMU Melaka. Our potential collaborators are MARII and car manufacturers such as Proton, Perodua etc. Besides, PEO-PMMA blend polymer batteries are investigated in SSE laboratory. In the project of PMMA−PEO blend solid polymer electrolytes, the XRD, FTIR, SEM and DSC results show clear evidence of the miscibility of PMMA and PEO. The incorporation of PMMA into PMMA−PEO blend polymer electrolytes shows significant improvement on ionic conductivity with lower concentration of PMMA used. Other research projects such as supercapacitors and UV avalanche photodiodes are developing in our laboratory since year 2000.
Battery Tester

Ts. Dr. You Ah Heng is an Associate Professor and Leader for Solid State Electronic Laboratory, Multimedia University, Melaka campus. His research interest includes thin avalanche photodiodes, polymer lithium ion batteries and optical fiber communication systems. He has been a principal investigator for various government agency research grants since 2000. He has published more than 60 international journal and conference papers. He was awarded the membership and Chartered Physicist (CPhys.) of Institute of Physics (IOP), UK since year 2000. He is a registered Professional Technologies with Malaysia Board of Technologies (MBOT) in year 2019. He is also the corporate member of Institute of Physics Malaysia (IFM).

